Use of fly ash and phosphogypsum for the synthesis of belite-sulfoaluminate clinker
S. Kramara*, L. Žibreta, E. Fidanchevskaa, V. Jovanovb, B. Angjushevab, V. Ducmana
a. Slovenian National Building and Civil Engineering Institute, (Ljubljana, Slovenia)
b. Ss. Cyril and Methodius University in Skopje, Faculty of Technology and Metallurgy, (Skopje, Macedonia)
*sabina.kramar@zag.si
| |
ABSTRACT
Fly ash and phosphogypsum were used as Naturally Occurring Radioactive Materials (NORM) by‑products for the synthesis of belite-sulfoaluminate clinkers. The influence of raw mixture composition and firing temperature was investigated. Clinkers and cements were examined by X‑ray powder diffraction and scanning electron microscopy with energy dispersive X‑ray spectroscopy. The compressive strength of the cements was determined after 28 days. Clinker phases identified included ye’elimite, ß‑phase of belite, ternesite and gehlenite, while the main hydration product of the cement pastes was ettringite. The results showed that belite-sulfoaluminate cements can be fabricated with a compressive strength of 45.9 N/mm2 by firing the raw mixture (70 wt.% marl, 10 wt.% bauxite and 20 wt.% phosphogypsum) at a temperature of 1320°C/1h.
|
| |
RESUMEN
Uso de cenizas volantes y fosfoyesos en la síntesis de clínkeres belíticos de sulfoaluminatos. En este estudio se han utilizado cenizas volantes y fosfoyeso como Naturally Occurring Radioactive Materials (NORM) para la síntesis de clínkeres belíticos de sulfoaluminatos. Se ha investigado la influencia de la composición de la materia prima y de las diferentes temperaturas de cocción. Los clínkeres y cementos se examinaron mediante difracción de rayos X y microscopía electrónica de barrido equipada con espectroscopía de energía dispersiva de rayos X. Los valores de compresión de los cementos se determinaron a la edad de 28 días. Las fases constituyentes de los clínkeres se identificaron como ye’elimita, fase-ß de la belita, ternesita y gehlenita, mientras que el principal producto de hidratación de la pasta de cemento se identificó como ettringita. Los resultados muestran que los cementos belíticos de sulfoaluminatos pueden ser fabricados con una resistencia a compresión de 45.9 N/mm2 mediante una cocción de la materia prima (70 % en peso de marga, 10 % de bauxita y 20 % de fosfoyeso) a una temperatura de 1320°C/1h.
|
1. INTRODUCTIONTOP
Ordinary Portland cement (OPC) is a widely used construction material that is manufactured via a highly energy-intensive process, producing large amounts of CO2 but also consuming large quantities of natural raw materials. Much effort has been made to reduce this CO2 footprint by utilising locally available secondary raw materials, replacing OPC clinker with mineral additives or the use of alternative fuels. Many studies have also focused on the development of new or alternative materials, one of which is belite-sulfoaluminate (BSA) cement. From an environmental and global warming perspective, such cements might reduce the impact caused by CO2 emissions not only due to their lower firing temperature (compared with that of OPC), but also due to the possibility of utilising alternative industrial waste and by-products as raw materials. BSA cement can be produced by combining various natural materials (limestone, clay, bauxite and gypsum) or by-products (fly ash, blast furnace slag, red mud, etc.) to provide the necessary CaO, SiO2, Al2O3 and SO3 required for phase formation (1–6). Additionally, BSA cements contain high amounts of sulphur, which makes high sulphur-containing waste materials such as phosphogypsum suitable for their production (7, 8).
Studies investigating the production of BSA cements from waste materials or by-products are increasingly commonplace (9–14). Gallardo et al. (15) reported the fabrication of sulfoaluminate belite clinker containing Ca4Al6O12SO4 as its main phase from the mixture of aluminium dross, fluorogypsum, fly ash and CaCO3, with heat treatment applied in the temperature range from 1100 to 1400°C. Three different cements were prepared using clinker powder mixed with 20 wt.% hemihydrate and various curing conditions. The main hydration phase was ettringite with typical acicular and hexagonal plates, with cement mechanical properties (compressive strengths of 40–47 MPa) found to be comparable to those of OPC. Ren et al. (16) investigated the potential production of sulfoaluminate cement (SAC) via a high-performance method using 100% industrial solid waste, with the aim of reducing energy and natural resource consumption. By combining coal gangue, flue gas desulfurisation, aluminium slag and carbide slag, sintered at 1100–1300°C, they obtained hydrated clinker specimens with compressive strengths of 59.5 MPa and 75 MPa after 3-day and 28-day curing, respectively. Belite-sulfoaluminate clinkers in general contain a minimum of 40–50 wt.% belite, 20–30 wt.% ye’elimite and ferrite phase, when the raw mixture is iron-rich (17); the complete phase assemblage can be formed at 1250°C (14, 17, 18). However, at enhanced firing temperatures the quantity of ye’elimite rises, which improves clinker reactivity. On the other hand, a high iron content can lower the optimum clinkering temperature (18), resulting in lower energy consumption. If an anhydrite surplus is available, the ternesite phase can be formed at slow cooling below 1200°C (5, 19–22). In high-aluminium clinkers in the presence of silicon, a nonreactive gehlenite phase can be formed (4, 13).
In cement pastes prepared from belite-sulfoaluminate clinker, a high early strength is ensured by the hydration of the ye’elimite phase and calcium sulfate source, from which large amounts of ettringite and aluminium hydroxide are produced (3, 23). Calcium sulfates can be sourced in cement as either as an anhydrite in the clinker, interground as gypsum after clinkering, or a combination of the two (1). After 28 days hydration, whereas the majority of the ye’elimite and calcium sulfate source will have reacted (24), the belite and ternesite phases react more slowly and contribute to the cement’s long-term strength (20). The reactivity of belite depends strongly on its polymorphism (25). Moreover, it has been reported that even after 90 days, unactivated β-belite may remain mostly unhydrated (24) and may start to react only after 1 year of hydration (2). On the other hand, Bullerjahn et al. (20) reported the precipitation of strätlingite after 48 hours hydration, initiated during belite and ternesite hydration. Ferrite and calcium sulfate can form ettringite, portlandite and amorphous iron hydroxide, which contribute, slightly, to the long-term strength of a cement (25).
Cementitious materials present a valuable target for valorising various wastes, residues and by-products (coal fly ash and bottom ash, slag from the iron and steel industry, red mud…), offering the potential to both meet environmental challenges and accelerate the pursuit of industrial sustainability (26). Depending on their content of natural radionuclides, some are listed as naturally occurring radioactive materials (NORMs). Industrial wastes and by-products are used not only in blended cements or as mineral additives in concrete, but may also be added during clinkerisation itself, partially or totally replacing the virgin raw materials. One solution for recycling NORMs such as fly ash and phosphogypsum is the synthesis of belite-sulfoaluminate cement clinkers (7, 11, 13, 14). Commonly, the concentration of radionuclides originating from such residues is decreased in the resulting products due to the dilution effect (26, 27). It is also expected that the trend of NORM recycling will continue with their use not only in concrete, but also in cement and clinker (26).
The aim of this study was to investigate the possibility of substituting natural raw materials with industrial NORM by-products such as phosphogypsum and fly ash, in the synthesis of belite-sulfoaluminate clinker. Thus, the phase composition and distribution in a series of belite-sulfoaluminate-based clinkers of different compositions were investigated at various heating profiles. In addition, the compressive strength and hydration products of cement pastes prepared from the clinkers were determined and discussed.
2. EXPERIMENTALTOP
2.1. Materials TOP
Clinker mixtures were prepared using natural raw materials (marl, bauxite) and two NORM by-products (phosphogypsum and siliceous fly ash). The chemical composition of the raw materials, given in Table 1, was determined using a Thermo Scientific ARL PERFORM’X Wavelength Dispersive X-Ray Fluorescence Spectrometer (WD XRF). Loss on ignition at 950°C was determined according to the standard EN 196-2, pt. 4.4.1 (28).
Table 1. Chemical composition of raw materials (wt.%).
| Parameter |
Marl |
Bauxite |
Fly ash |
Phosphogypsum |
| SiO2 |
14.19 |
5.60 |
58.90 |
2.32 |
| CaO |
38.52 |
0.35 |
4.33 |
32.40 |
| Al2O3 |
5.04 |
87.20 |
20.80 |
0.18 |
| Fe2O3 |
2.59 |
1.53 |
7.13 |
0,12 |
| MgO |
2.39 |
0.22 |
2.11 |
< 0.05 |
| K2O |
0.68 |
0.23 |
2.58 |
0.03 |
| Na2O |
0.04 |
b.d.l. |
0.65 |
< 0.03 |
| SO3 |
0.21 |
b.d.l. |
0.90 |
43.00 |
| P2O5 |
0.02 |
0.24 |
0.23 |
0.13 |
| TiO2 |
0.19 |
2.91 |
0.76 |
0.04 |
| LOI |
35.37 |
0.16 |
1.48 |
19.50 |
2.2. Preparation of raw mixturesTOP
Three mixtures with different compositions were prepared by substituting bauxite with fly ash (A - marl, bauxite, phosphogypsum;
B - marl, bauxite/fly ash, phosphogypsum; C - marl, fly ash, phosphogypsum); the amount of phosphogypsum remained constant in all mixtures (Table 2).
Table 2. Clinker mixtures (wt.%).
| Raw mixture |
Marl |
Bauxite |
Fly ash |
Phosphogypsum |
| A |
70 |
10 |
- |
20 |
| B |
70 |
5 |
5 |
20 |
| C |
70 |
- |
10 |
20 |
The raw mixtures were homogenised and ground in a planetary mill, before being passed through a 125 μm sieve. A total of 350
g of material was prepared for each mixture. Two pressed pellets of 30 g each were prepared from each mixture using a Weber Pressen KIP 100 at 15 MPa. The clinker mixtures were subjected to the following heating regimes: (a) heating to 1120°C (T1), 1220°C (T2) or 1320°C (T3), (b) heating rate: 3°C/min, (c) holding time at the final temperature for 1 h and (d) natural cooling in a closed furnace.
The chemical composition of raw mixtures before and after clinkering is shown in Tables 3 and 4, respectively. Samples were analysed using a Thermo Scientific ARL PERFORM’X Wavelength Dispersive X-Ray Fluorescence Spectrometer
(WD XRF). Loss on ignition at 950°C was determined according to the standard EN 196-2, pt. 4.4.1 (28).
Table 3. Chemical composition of raw mixtures.
| Mixture |
A |
B |
C |
| SiO2 |
12.34 |
14.95 |
17.61 |
| CaO |
32.05 |
32.90 |
33.21 |
| Al2O3 |
13.83 |
9.79 |
6.10 |
| Fe2O3 |
1.80 |
2.09 |
2.38 |
| MgO |
1.99 |
2.02 |
2.16 |
| K2O |
0.49 |
0.61 |
0.73 |
| Na2O |
0.27 |
0.21 |
0.29 |
| SO3 |
6.21 |
6.40 |
6.43 |
| P2O5 |
0.10 |
0.13 |
0.08 |
| MnO |
0.05 |
0.06 |
0.06 |
| TiO2 |
0.49 |
0.37 |
0.23 |
| LOI |
29.27 |
29.46 |
29.52 |
Table 4. Chemical composition of raw mixtures heated at different temperatures.
| Mixture |
A |
B |
C |
| T (°C) |
1120 |
1220 |
1320 |
1120 |
1220 |
1320 |
1120 |
1220 |
1320 |
| SiO2 |
17.62 |
17.64 |
17.59 |
21.31 |
21.40 |
22.23 |
25.18 |
24.86 |
25.99 |
| CaO |
45.82 |
46.20 |
45.49 |
46.79 |
47.51 |
48.80 |
47.30 |
46.84 |
49.75 |
| Al2O3 |
19.06 |
19.56 |
19.93 |
13.91 |
14.05 |
14.56 |
8.88 |
9.06 |
9.18 |
| Fe2O3 |
2.54 |
2.61 |
2.59 |
2.98 |
3.05 |
3.13 |
3.38 |
3.31 |
3.49 |
| MgO |
2.65 |
2.70 |
2.58 |
2.86 |
2.93 |
2.99 |
3.06 |
3.11 |
3.24 |
| K2O |
0.69 |
0.69 |
0.54 |
0.87 |
0.89 |
0.73 |
1.03 |
1.04 |
1.12 |
| Na2O |
0.17 |
0.17 |
b.d.l. |
0.22 |
b.d.l. |
0.24 |
0.31 |
0.44 |
0.59 |
| SO3 |
8.67 |
8.24 |
6.24 |
8.85 |
7.97 |
5.14 |
8.77 |
7.81 |
4.36 |
| P2O5 |
0.13 |
0.21 |
0.13 |
0.14 |
0.13 |
0.19 |
0.13 |
0.13 |
0.15 |
| MnO |
0.07 |
0.07 |
0.07 |
0.08 |
0.08 |
0.08 |
0.09 |
0.09 |
0.09 |
| TiO2 |
0.72 |
0.73 |
0.73 |
0.53 |
0.53 |
0.57 |
0.35 |
0.33 |
0.38 |
| LOI |
0.19 |
0.19 |
0.26 |
0.11 |
0.19 |
0.23 |
0.10 |
0.25 |
0.26 |
After firing, all clinkers were ground to below 125 μm and the cements prepared by adding 10 wt.% gypsum (chemical grade, with a min. purity of 99%, Kemika, Zagreb). Finally, the cement pastes were produced using a water/cement ratio of 0.6.
2.3. MethodsTOP
The content of free lime (f-CaO) in the clinker samples was determined chemically after dissolving in ethanol-glycol (29).
The mineral composition of the clinkers and prepared cement pastes (after 28 days curing) was determined via X-ray diffraction in a PANalytical Empyrean X-ray diffractometer equipped with CuKα radiation at λ = 1.54 A. Samples were milled to a particle size of less than 50 μm. Data were collected at 45 kV and a current of 40 mA, over the 2θ range from 4° to 70°, at a scan rate of 0.026° 2θ/min. The obtained data were then analysed in the X’PertHighScore Plus diffraction software from PANalytical, using PAN-ICSD V3.4 powder diffraction files. Hydration processes were inhibited by solvent exchange, where crushed samples were immersed in 100 ml of isopropanol for 15 min. Finally, the samples were dried at 70°C for 60 min.
Uncoated polished cross-sections of clinker samples were examined using a JEOL 5500 LV Scanning Electron Microscope (SEM)
equipped with an Energy Dispersive X-ray spectrometer (EDS), at an accelerating voltage of 20 kV and working distance of 20
mm.
For the determination of cement compressive strength, the mixtures were cast into prismatic moulds of dimensions 10 × 10 ×
25 mm. The cement paste samples were demoulded 24 h after casting and cured until testing in sealed plastic bags under laboratory conditions at 21±2°C. Compressive strength was determined after 28 days in two specimens per mixture using the ToniNORM Toni-Technic testing machine (Zwick) at a loading rate of 0.04 kN/s.
3. RESULTS AND DISCUSSIONTOP
As each of the three raw clinker mixtures in Table 2 were fired at three different temperatures, the obtained clinkers were further characterised regarding their phase composition and microstructure.
3.1. Clinker characterisationTOP
3.1.1. Clinker mineralogy and f-CaO contentTOP
Clinker f-CaO content (Table 5) did not exceed 0.23%, indicating that the available Ca in the raw mixtures was consumed during the burning process.
Table 5. f-CaO content of clinker samples.
| Mixture |
A |
B |
C |
| T (°C) |
1120 |
1220 |
1320 |
1120 |
1220 |
1320 |
1120 |
1220 |
1320 |
| f-CaO (%) |
0.13 |
0.11 |
0.11 |
0.10 |
0.23 |
0.18 |
0.27 |
0.13 |
0.11 |
Table 6 displays the results of X-ray powder diffraction analysis for clinker mixtures A (no substitution of bauxite with fly ash), B (half of bauxite substituted with fly ash) and C (bauxite fully substituted with fly ash) fired at the three different temperatures, with the corresponding X-ray powder diffraction patterns shown in Figure 1. In all three mixtures the presence of the β phase of belite (β-C2S), orthorhombic and cubic ye’elimite (C4A3S), ternesite (C5S2S), gehlenite (C2AS), ferrite (C4AF) and anhydrite (CS) was evident.
Table 6. Clinker phase composition, as obtained by X–ray powder diffraction. Legend: - = undetected; tr = trace; + = low proportion;
++ = medium proportion; +++ = high proportion; ++++ = predominant phase.
| Clinker Phase |
A |
B |
C |
| |
1120°C |
1220°C |
1320°C |
1120°C |
1220°C |
1320°C |
1120°C |
1220°C |
1320°C |
| β-belite |
tr/+ |
+ |
++ |
+ |
+ |
++ |
+ |
+ |
++ |
| Ye’elimite |
++ |
+++ |
++++ |
++ |
++ |
+++ |
+ |
tr/+ |
++ |
| Ternesite |
++ |
++ |
- |
++ |
++ |
- |
+++ |
+++ |
- |
| Gehlenite |
++ |
++ |
- |
++ |
++ |
- |
+++ |
+++ |
- |
| Ferrite |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
| Anhydrite |
tr |
tr |
+ |
+ |
tr/+ |
+ |
+ |
+ |
+ |
| Periclase |
+ |
tr |
tr/+ |
tr |
tr |
tr |
- |
tr |
tr |
| Free-lime |
tr |
tr |
tr |
tr |
tr |
tr |
+ |
tr/+ |
+ |
| Quartz |
+ |
+ |
- |
+ |
+ |
- |
+ |
+ |
- |
|
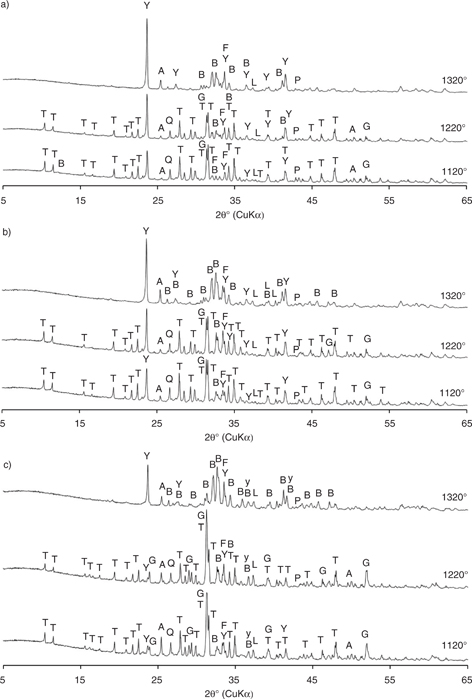 Figure 1. X-ray patterns of clinkers fired at 1120°C, 1220°C and 1320°C: a) Mixture A; b) Mixture B; c) Mixture C. Labels for clinker phases: B = β-belite; Y = ye’elimite; F = ferrite; G = gehlenite; T = ternesite; A = anhydrite; Q = quartz; L = lime. Figure 1. X-ray patterns of clinkers fired at 1120°C, 1220°C and 1320°C: a) Mixture A; b) Mixture B; c) Mixture C. Labels for clinker phases: B = β-belite; Y = ye’elimite; F = ferrite; G = gehlenite; T = ternesite; A = anhydrite; Q = quartz; L = lime.
|
|
As can be seen in Figure 1, the amount of ye’elimite and belite in mixtures A and B increased with firing temperature. However, mixture A was characterised by a higher ye’elimite content and lower belite content than mixture B. The presence of ternesite was evident at 1120°C and 1220°C, but was completely absent at a clinkering temperature of 1320°C due to its decomposition into belite and anhydrite (7, 30). Ternesite is stable in the temperature range of 900–1200°C and is formed by reacting belite and anhydrite (31), a fact supported by the present study in which a slight increase in anhydrite content with firing temperature was observed. Additional phases recorded included gehlenite, which formed only at clinkering temperatures of 1120°C and 1220°C, and ferrite, which was identified in both clinkers at all three clinkering temperatures. As a remnant of the raw material, quartz was present in smaller amounts in clinkers heated at 1120°C and 1220°C. This indicates that the raw material had not reacted completely at 1220°C, but had done so at 1320°C. The presence of anhydrite at all firing temperatures suggests that more SO3 was present than was needed for the formation of ye’elimite (6).
Whereas only a very low amount of ye’elimite was observed in clinker mixture C heated at temperatures of 1120°C and 1220°C, levels increased significantly at 1320°C. Clinker mixture C was also characterised by a high amount of belite compared to the other two mixtures, increasing with rising clinkering temperature. As was observed for mixtures A and B, ternesite and gehlenite were present in clinker mixture C at temperatures of 1120°C and 1220°C. However, gehlenite levels were much higher in mixture C due to the large amount of silica found in the raw fly ash (Table 1). Ferrite was present at all three clinkering temperatures, while the amount of anhydrite in clinker mixture C decreased with increasing firing temperature. A small amount of quartz was found only in clinkers heated at 1120°C and 1220°C.
The selected raw materials had a significant influence on the formation of major clinker phases. Clinker mixture A exhibited the best development of the ye’elimite phase at all three clinkering temperatures due to its high alumina content. In mixture B the ye’elimite phase was also clearly expressed, but at a lower ratio to belite with respect to mixture A. Mixture C, in which bauxite was completely replaced by fly ash, was characterised by the lowest amounts of the ye’elimite phase due to the associated alumina deficit. However, levels significantly increased at 1320°C, as this temperature was sufficient for the conversion of anhydrite and gehlenite into belite and ye’elimite (32). As a result, samples also exhibited decreased anhydrite and an absence of gehlenite at this temperature. The presence of anhydrite in all clinker samples indicates a surplus of SO3 in the raw mixtures. Consequently, at clinkering temperatures of 1120°C and 1220°C the reaction between belite and anhydrite led to the formation of ternesite (17, 18, 20). Indeed, when both SiO2 and SO3 are high in the raw mix, ternesite forms easily (6). At 1320°C ternesite was not observed, while the amount of belite was at its highest among the three firing temperatures. This pattern can be explained by the decomposition of ternesite at temperatures above 1250°C (31, 33, 34). Belite and ternesite phases were dominant in clinker mixture C, where the addition of fly ash provided larger quantities of silica. A clearly detectable amount of ferrite was evident in the two clinkers with added fly ash (mixtures B and C), due to the relatively high iron content of this raw material. Gehlenite was recorded together with ternesite at clinkering temperatures of 1120°C and 1220°C in all mixtures, with levels of both gradually increasing with a higher amount of fly ash in the raw mixture, i.e. from mixtures A to C. Neither ternesite nor gehlenite were observed at a temperature of 1320°C.
3.1.2. Clinker microstructure TOP
The phase distribution and microstructure of the clinkers were studied by SEM/EDS analysis.
According to this analysis, the clinkers of all three compositions heated at the lower clinkering temperatures (1120°C and
1220°C) were highly porous and heterogeneous (Figure 2). Clinkerisation at 1220°C resulted in a slightly denser and semi‑homogeneous texture, with a higher degree of crystallisation. However, phase composition was the same at both temperatures, with ternesite, belite, ye’elimite, gehlenite, ferrite and anhydrite all observed. Mixtures A and B were found to contain clusters comprising round grains of aluminium oxide, as well as interstitial phases consisting of gehlenite and ferrite (Figure 3), neither of were present at 1320°C, as sintering proceeded. The grains were absent in clinker mixture C, due to the depletion of alumina in these samples and the incorporation of siliceous fly ash. Instead, ternesite formed clear anhedral grains, with laths of gehlenite clearly visible.
|
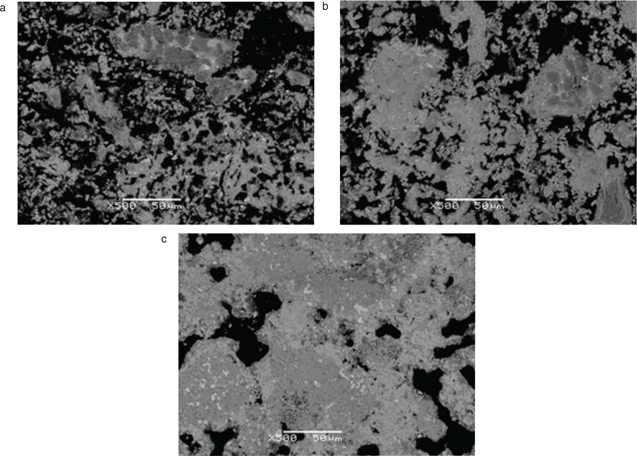 Figure 2. SEM/BSE photomicrographs showing the microstructural densification of clinker mixture A at sintering temperatures of (a) 1120°C, (b) 1220°C and (c) 1320°C. Figure 2. SEM/BSE photomicrographs showing the microstructural densification of clinker mixture A at sintering temperatures of (a) 1120°C, (b) 1220°C and (c) 1320°C.
|
|
|
 Figure 3. SEM/BSE photomicrograph of clinker mixture A heated at 1220°C, showing rounded Al‑enriched crystals. The interstitial phase consists of gehlenite (G) and ferrite (F). Figure 3. SEM/BSE photomicrograph of clinker mixture A heated at 1220°C, showing rounded Al‑enriched crystals. The interstitial phase consists of gehlenite (G) and ferrite (F).
|
|
At 1320°C, clinker texture was homogeneous with low porosity due to the progress of the sintering process (Figure 4). Ye’elimite and belite were the dominant phases, with the ferrite phase appearing as individual smaller grains. The amount of ye’elimite decreased clearly with a higher content of fly ash in the raw mixture. As determined by SEM/EDS, higher amounts of iron were incorporated in the ye’elimite phase of mixtures B and C, reflecting the introduction of iron-containing fly ash in the raw mixtures. Such incorporation can lead to iron solid solutions of the ye’elimite phase, which may in turn influence cement hydration kinetics (35, 36). In contrast, well‑developed belite crystals were predominant in clinker mixture C. Ternesite and gehlenite were not observed at this temperature, as also identified by X‑ray powder diffraction.
|
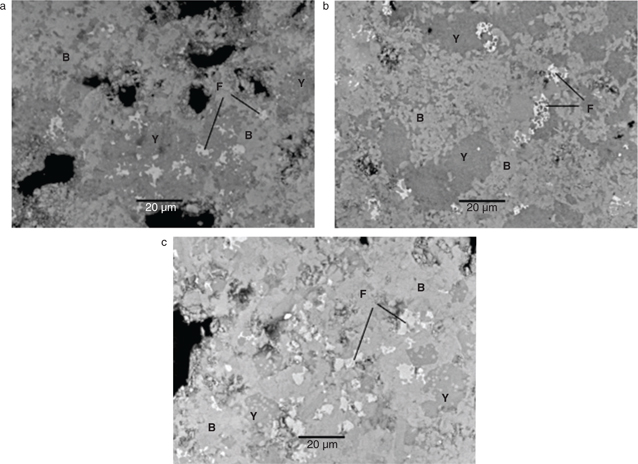 Figure 4. SEM/BSE photomicrographs of the polished sections of clinkers heated at 1320°C. Microstructure of (a) clinker mixture A, (b)
clinker mixture B and (c) clinker mixture C. Phases identified include belite (B), ye’elimite (Y) and ferrite (F). Figure 4. SEM/BSE photomicrographs of the polished sections of clinkers heated at 1320°C. Microstructure of (a) clinker mixture A, (b)
clinker mixture B and (c) clinker mixture C. Phases identified include belite (B), ye’elimite (Y) and ferrite (F).
|
|
3.2. Characterisation of cement pastesTOP
Cements were prepared by adding 10 wt. % of gypsum to the ground clinker, with the cement pastes produced using a water/cement ratio of 0.6. Hardened pastes were then characterised in terms of their phase compositions and mechanical properties (compressive strength).
3.2.1. Phase composition of hydrated cements TOP
Figure 5 displays the phase composition of the cement pastes, prepared from the studied clinkers, after 28 days curing. The main hydration product was ettringite (C6AS3H32), followed by unreacted gypsum (CSH) in varying amounts, as well as remnant clinker phases including belite (β-C2S), ternesite (C5S2S), gehlenite (C2AS) and ye’elimite (C4A3S).
|
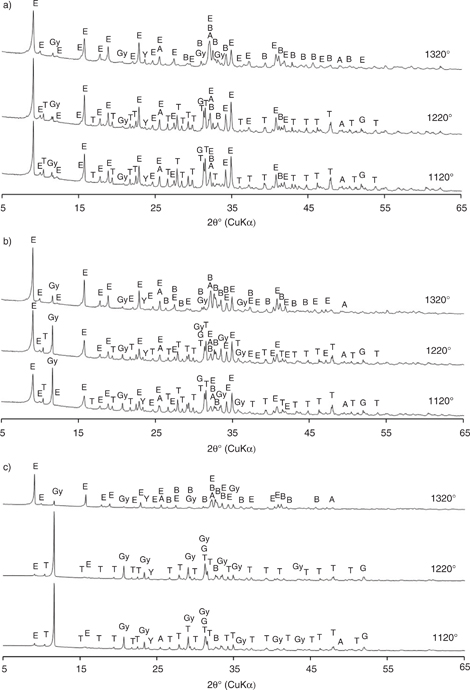 Figure 5. X-ray diffraction patterns of cement hydration products after 28 days for clinkers produced from: a) mixture A, b) mixture B and c) mixture C. Labels for clinker phases: E = ettringite; B = β-belite; Y = ye’elimite; F = ferrite; G = gehlenite; T = ternesite; A = anhydrite; Gy = gypsum. Figure 5. X-ray diffraction patterns of cement hydration products after 28 days for clinkers produced from: a) mixture A, b) mixture B and c) mixture C. Labels for clinker phases: E = ettringite; B = β-belite; Y = ye’elimite; F = ferrite; G = gehlenite; T = ternesite; A = anhydrite; Gy = gypsum.
|
|
Regarding the cement pastes based on clinker mixture A (Figure 5a), ye’elimite was barely detected in all three samples, indicating that almost all ye’elimite was consumed in the formation of ettringite (23). In addition, almost all gypsum was consumed during the hydration process. A similar situation was observed for the cement pastes produced from clinker mixture B (Figure 5b), in which a gradual increase in the intensity of ettringite was recorded with clinkering temperature. This result is in accordance with the increasing amount of ye’elimite in these clinkers (Figure 1b). Ye’elimite was also barely detected in all three hydrated samples, indicating a high degree of hydration. The latter is additionally confirmed by the decreasing amount of gypsum found in the cement pastes containing a higher amount of ettringite. During 28 days hydration of the cement pastes based on clinker mixture C heated to 1120°C and 1220°C, barely perceptible amounts of ettringite were formed (Figure 5c), likely related to the very low amounts of ye’elimite present in the clinkers. Furthermore, in the cement paste prepared from clinker mixture C heated to 1320°C, almost all ye’elimite was hydrated to ettringite. Only a small amount of gypsum remained in this sample, in contrast to the large amounts found in the pastes prepared from clinkers heated at the two lower temperatures, reflecting the low levels of reactive phases identified in these two clinkers. Belite, ternesite, gehlenite and anhydrite were all present as remnant clinker phases in the cement pastes.
It is evident that after 28 days curing, most of the available ye’elimite in the cement pastes had reacted with the gypsum and anhydrite to form ettringite, which defines the early strength of the cement. The greatest amount of ettringite was identified in cements derived from clinker mixture A, followed by mixtures B and C; gypsum levels increased accordingly. The noticeable amount of ternesite in the cement pastes indicates that the degree of ternesite consumption after 28 days was very low, a result also reported in other studies (20). A similar situation was observed for belite. The presence of anhydrite and gypsum even after 28 days suggests that these minerals would continue to be available to react with ferrite, slightly enhancing cement compressive strength (3). Gehlenite was found in all cement pastes made from low-temperature clinkers (1120°C and 1220°C), potentially forming strätlingite during the later stages of the hydration process (4).
3.2.2. Compressive strength TOP
Table 7 displays the compressive strength of the cement pastes obtained after 28 days curing. Cement paste compressive strength depends on the raw material design of the clinker mixtures, firing temperature and consequent clinker composition.
Table 7. Compressive strength (N/mm2) of cement pastes after 28 days curing.
| Mixture |
A |
B |
C |
| T (°C) |
1120 |
1220 |
1320 |
1120 |
1220 |
1320 |
1120 |
1220 |
1320 |
| Compressive strength (N/mm2) |
14.8 |
18.3 |
45.8 |
5.9 |
3.5 |
15.9 |
0.5 |
0.2 |
1.6 |
The cement paste based on clinker mixture A (no substitution of bauxite with fly ash) heated at 1320°C presented the greatest compressive strength of 45.8 N/mm2. This result is closely related to the high levels of the hydration product ettringite found in this particular paste, which in turn reflect the presence of ye’elimite in the clinker mixture. At lower heating temperatures of 1120 and 1220°C, lower values of compressive strength were obtained, at 14.80 and 18.30 N/mm2, respectively. Similarly, the compressive strength of the cement paste composed of clinker mixture B (half of bauxite substituted with fly ash) heated at 1320°C was higher than that of the lower-temperature clinker mixtures, at 15.90 N/mm2. The cement pastes based on clinker mixture C (bauxite fully substituted with fly ash) presented the lowest compressive strength of all the tested samples (0.2 to 1.6 N/mm2). For the pastes derived from clinkers heated at 1120°C and 1220°C, this weak compressive strength is related to the low amounts of ettringite in the pastes (Figure 5) and low ye’elimite levels in the corresponding clinkers (Figure 1, Table 6). However, it is interesting to note that the compressive strength of the cement paste based on clinker mixture C heated at 1320°C was also low, despite the fact that this paste’s ettringite content was comparable to that of the cement pastes based on clinker mixtures A and B (heated at 1320°C). One potential explanation for this result could be delayed ettringite formation (37, 38, 39). Alternatively, according to Idrissi et al. (36), doping calcium sulfoaluminate with iron seems to slightly reduce the dissolution rate and also the nucleation rate during the induction period. Nevertheless, further study is required to explain this issue.
Generally, the highest values of compressive strength were obtained for the three clinker mixtures subjected to the highest temperature, i.e. 1320°C, a result in agreement with similar studies reported in the literature (15). The lower compressive strength of the cement pastes composed of clinker mixtures heated at 1120 and 1220°C is the result of their phase composition, which did not contribute to cement mechanical properties during the selected curing period of
28 days. Indeed, ternesite hydration is typically very slow and can contribute to a decrease in early strength (7). This is also the case for belite, which is usually present in calcium sulfoaluminate cements, as its hydration is also typically slow but provides long-term strength (1). Furthermore, the poor hydraulic ability of gehlenite means that it contributes little to the compressive strength of a cement (11). The slight decrease in compressive strength of the cement pastes based on clinker mixtures B and C heated at 1220°C is thus most probably the result of the higher quantity of phases that do not influence cement strength, such as gehlenite and ternesite. These phases were not found in the clinker mixtures obtained at 1320°C. After 28 days hydration, cement compressive strength was mainly conditioned by the available ye’elimite and gypsum or anhydrite.
4. CONCLUSIONSTOP
The present work focuses on the synthesis of belite–sulfoaluminate clinkers incorporating NORM by-products (phosphogypsum and siliceous fly ash). The study characterises the clinkering products obtained at three temperatures (1120, 1220 and 1320°C/1h), as well as the strength development and hydration behaviour of cement pastes produced using these clinkers.
The main conclusions can be summarised as follows:
| • |
The main clinker phase was highly reactive ye’elimite, followed by slowly reactive belite and ternesite. Ye’elimite and belite content increased with firing temperature. Only a small amount of ye’elimite was detected in clinker mixture C fired at lower temperatures. In samples containing fly ash, a higher amount of slowly reactive gehlenite phase was present. |
| • |
Ye’elimite levels decreased in clinkers with greater bauxite substitution for fly ash, whereas belite and gehlenite content increased accordingly. |
| • |
The main hydration product was ettringite, the presence of which was closely related to that of ye’elimite in the clinkers. A higher ye’elimite content in the clinkers resulted in increased ettringite levels and consequently the greater compressive strength of the hydrated paste. |
| • |
The highest compressive strength values after 28 days curing were achieved in cement pastes produced using clinkers with the highest firing temperature, i.e. 1320°C. Clinker mixtures containing a higher amount of fly ash instead of bauxite resulted in lower values of compressive strength (A>>>B>C). The lower Al2O3 content of these mixtures was not sufficient for ye’elimite formation and contributed to the depletion of reactive phase formation, i.e. ye’elimite and consequently ettringite. |
| • |
The greatest compressive strength of 45.8 N/mm2 was achieved using clinker heated at 1320°C/1h and containing 20 wt.% phosphogypsum as the NORM representative. In contrast, the use of clinker heated at the same temperature and with the same phosphogypsum content, but substituting 5 wt.% bauxite with fly ash, resulted in a decrease in compressive strength to 15.9 N/mm2. |
The present study provides preliminary results regarding the potential use of NORM by‑products in the synthesis of environmentally friendly belite-sulfoaluminate clinkers. Based on this research it seems that whereas phosphogypsum could be implemented into BSA cement production, siliceous fly ash addition would not contribute positively (at least under the conducted experimental conditions) to early cement mechanical properties. However, due to the tested mixtures’ high belite content, which hydrates more slowly and thus contributes to the long‑term mechanical strength of the resulting cement, future work will focus on studying the long‑term performance of belite-sulfoaluminate cements, mixture optimisation and the radiological properties of the final products.
ACKNOWLEDGEMENTSTOP
Project No. C3330-17-529035 “Raziskovalci-2.0-ZAG-529035” was granted by Ministry of Education, Science and Sport of Republic of Slovenia. The investment is co-financed by the Republic of Slovenia, Ministry of Education, Science and Sport and the European Regional Development Fund. The work was also financially supported by COST Action TU 1301: “NORM for Building materials–NORM4BUILDING” (www.norm4building.org). The Metrology Institute of the Republic of Slovenia is acknowledged for the use of XRF. The authors thank Mojca Škerl and Dušica Tauzes for their help with sample preparation and analysis, as well as NOVA Refractories A.D. Pehchevo, Macedonia.
REFERENCESTOP
| 1. |
Glasser, F.P.; Zhang, L. (2001) High-performance cement matrices based on calcium sulfoaluminate-belite compositions. Cem. Concr. Res. 31, 1881–1886. https://doi.org/10.1016/S0008-8846(01)00649-4. |
| 2. |
Cuberos, A.J.M; De La Torre, A.G.; Alverez-Pinazo, G.; Martin-Sedeño, M.C.; Schollbach, K.; Pollman, H.; Aranda, M.A.G. (2010) Active Iron-Rich Belite Sulfoaluminate Cements: Clinkering and Hydration. Environ. Sci. Technol. 44, 6855–6862. https://doi.org/10.1021/es101785n. |
| 3. |
Chen, I.A.; Juenger, M.C.G. (2011) Synthesis and hydration of calcium sulfoaluminate-belite cements with varied phase composition. J. Mater. Sci. 46, 2568–2577. https://doi.org/10.1007/s10853-010-5109-9. |
| 4. |
Palou, M.; Majling, J.; Doval, M.; Kozankova, J.; Mojumdar, S.C. (2005) Formation and Stability of Crystallohydrates in the Non-equilibrium System During Hydration of SAB Cements. Ceramics-Silicáty 49 [4], 230–236. |
| 5. |
Strigač, J.; Palou, M.T.; Krištin, J.; Majling, J. (2000) Morphology and Chemical Composition of Minerals Inside the Phase Assemblage C-C2S-C4A3S-C4AF-CS Relevant to Sulphoaluminate Belite Cements. Ceramics-Silicáty 44 [1], 26–34. |
| 6. |
Roy, D.M.; Silsbee, M.R.; Xie, Z. (1999) Influences of Surplus SO3 in FBC Ash on Formation of Belite-Rich Sulfoaluminate Clinkers, International Ash Utilization Symposium, Center for Applied Energy Research, University of Kentucky, paper#30. |
| 7. |
Shen, Y.; Qian, J.; Huang, Y.; Yang, D. (2015) Synthesis of belite sulfoaluminate-ternesite cements with phosphogypsum. Cement Concr. Compos. 63, 67–75. https://doi.org/10.1016/j.cemconcomp.2015.09.003. |
| 8. |
Ukrainczyk, N.; Frankovič Mihelj, N.; Šipušič, J. (2013) Calcium Sulfoaliminate Eco-Cement from Industrial Waste. Chem. Biochem. Eng.Q. 27 [1], 83–93. http://hrcak.srce.hr/99441. |
| 9. |
El-Alfi, E.A.; Gado, R.A. (2016) Preparation of calcium sulfoaluminate-belite cement from marble sludge waste. Constr. Build. Mater. 113, 764–772. https://doi.org/10.1016/j.conbuildmat.2016.03.103. |
| 10. |
Rungchet, A.; Chindraprasirt, P.; Wansom, S.; Pimraksa, K. (2016) Hydrothermal synthesis of calcium sulfoaluminate-belite cement from industrial waste materials. J. Clean. Prod. 115, 273–283. https://doi.org/10.1016/j.jclepro.2015.12.068. |
| 11. |
Wang, W.; Wang, X.; Zhu, J.; Wang, P.; Ma, C. (2013) Experimental Investigation and Modeling of Sulfoaluminate Cement Preparation Using Desulfurization Gypsum and Red Mud. Ind. Eng. Chem. Res. 52, 1261–1266. https://doi.org/10.1021/ie301364c. |
| 12. |
Jewell, R.B.; Rathbone, R.F.; Duvallet, T.Y.; Robi, T.L.; Mahboub, K.C. (2015) Fabrication and Testing of Low-Energy Calcium Sulfoaluminate-Belite Cements that Utilize Circulating Fluidized Bed Combustion By-Products. Coal Combustion and Gasification Products Journal. 7, 9–18. https://doi.org/10.4177/CCGP-D-15-00001.1. |
| 13. |
Arjunan, P.; Silsbee, R.M.; Roy, D.M. (1999) Sulfoaluminate-belite cement from low-calcium fly ash and sulfur-rich and other industrial by-products. Cem. Concr. Res. 29, 1305–1311. https://doi.org/10.1016/S0008-8846(99)00072-1. |
| 14. |
Ma, B.; Li, X.; Mao, Y.; Shen, X. (2013) Synthesis and characterization of high belite sulfoaluminate cement through rich alumina fly ash and desulfurization gypsum. Ceramics – Silikáty. 57 [1], 7–13. |
| 15. |
Gallardo, M.; Almanza, J.M.; Cortés, D.A.; Escobedo, J.C.; Escalante-García, J.I. (2014) Synthesis and mechanical properties of a calcium sulphoaluminate cement made of industrial wastes. Mater. Construc. 64 [315], 1–8 https://doi.org/10.3989/mc.2014.04513. |
| 16. |
Ren, C.; Wang, W.; Li, G. (2017) Preparation of high-performance cementitious materials from industrial solid waste. Constr. Build. Mater. 152, 39–47. https://doi.org/10.1016/j.conbuildmat.2017.06.124. |
| 17. |
Álvarez-Pinazo, G.; Cuesta, A.; García-Maté, M.; Santacruz, I.; Losilla, E.R.; Dela Torre, A.G.; León-Reina, L.; Aranda, M.A.G. (2012) Rietveld quantitative phase analysis of yeelimite-containing cements. Cem. Concr. Res. 42 [7], 960–971. https://doi.org/10.1016/j.cemconres.2012.03.018. |
| 18. |
Martín-Sedeño, M.C.; Cuberos, A.J.M.; De la Torre, Á.G.; Álvarez-Pinazo, G.; Ordónez, L.M.; Gateshki, M.; Aranda, M.A.G. (2010) Aluminum-rich belite sulfoaluminate cements: Clinkering and early age hydration. Cem. Concr. Res. 40, 359–369. https://doi.org/10.1016/j.cemconres.2009.11.003. |
| 19. |
Beretka, J.; de Vito, B.; Santoro, L.; Sherman, N.; Valenti, G.L. (1993) Utilisation of industrial wastes and by-products for the synthesis of special cements. Resour. Conserv. Recy. 9, 179–190. https://doi.org/10.1016/0921-3449(93)90002-W. |
| 20. |
Bullerjahn, F.; Schmitt, D.; Haha, M.B. (2014) Effect of raw mix design and clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 59, 87–95. https://doi.org/10.1016/j.cemconres.2014.02.004. |
| 21. |
Bullerjahn, F.; Zajac, M.; Ben Haha, M. (2014) CSA raw mix design: effect on clinker formation and reactivity. Mater. Struct. 48 [12], 3895–3911. https://doi.org/10.1617/s11527-014-0451-z. |
| 22. |
Hanein, T.; Galanb, I.; Glasser, F.P.; Skalamprinos, S.; Elhoweris, A.; Imbabi, M.S.; Bannerman, M.N. (2017) Stability of ternesite and the production at scale of ternesite-based clinkers. Cem. Concr. Res. 98, 91–100. https://doi.org/10.1016/j.cemconres.2017.04.010. |
| 23. |
Kasselouri, V.; Tsakiridis, P. (1995) A study on the hydration products of a non-expensive sulfoaluminate cement. Cem. Concr. Res. 25 [8], 1726–1736. https://doi.org/10.1016/0008-8846(95)00168-9. |
| 24. |
Chen, I.A.; Juenger, M.C.G. (2012) Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cement Concr. Compos. 34, 893–902. https://doi.org/10.1016/j.cemconcomp.2012.04.006. |
| 25. |
Álvarez-Pinazo, G.; Santacruz, I.; Leon-Reina, L.; Aranda, M.A.G.; De la Torre, A.G. (2013) Hydration Reactions and Mechanical Strength Developments of IronRich Sulfobelite Eco-cements. Ind. Eng. Chem. Res. 52, 16606–16614. https://doi.org/10.1021/ie402484e. |
| 26. |
J. Labrincha, F. Puertas, W. Schroeyers, K. Kovler, Y. Pontikes, C. Nuccetelli, P. Krivenko, O. Kovalchuk, O. Petropavlovsky, M. Komljenovic, E. Fidanchevska, R. Wiegers, E. Volceanov, E. Gunay, M.A. Sanjuan, V. Ducman, B. Angjusheva, D. Bajare, T. Kovacs, G. Bator, S. Schreurs, J. Aguiar, J.L. Povis (2017) From NORM by-products to building materials, In Schroeyers, W. (ed) Naturally Occurring Radioactive Materials in Construction, Integrating Radiation Protection in Reuse (COST Action Tu1301 NORM4BUILDING).Woodhead Publishing Series in Civil and Structural Engineering, Elsevir. |
| 27. |
Saadaoui, E.; Ghazel, N.; Romdhane, C.B.; Massoudi, N. (2017) Phosphogypsum: potential uses and problems-a review, International Journal of Environmental studies, 1–10. https://doi.org/10.1080/00207233.2017.1330582. |
| 28. |
EN 196-2:2013, Method of testing cement. Chemical analysis of cement. |
| 29. |
Javellana, M.; Jawed, I. (1982) Extraction of free lime in Portland cement and clinker by ethylene glycol. Cem. Concr. Res. 12, 399–403. https://doi.org/10.1016/0008-8846(82)90088-6. |
| 30. |
Galan, I.; Hanein, T., Elhoweris, A.; Bannerman, M.N.; Glasser, F.P. (2017) Phase Compatibility in the System CaO-SiO2-Al2O3-SO3-Fe2O3 and the Effect of Partial Pressure on the Phase Stability. Industrial & Engineering Research. 56, 2341–2349. https://doi.org/10.1021/acs.iecr.6b03470. |
| 31. |
Marroccoli, M.; Pace M.L.; Telesca, A.; Valenti, G.L. (2010) Synthesis of calcium sulfoaluminate cements from Al2O3-rich by-products from aluminum manufacture, In: Proceedings of the 2ed International Congress on Sustainable Construction Materials and Technologies, Ancona, Italy, 2010. |
| 32. |
Jen, G.; Skalamprinos, S.; Whittaker, M.; Galan, I.; Ibabai, M.S.; Glasser, F.P. (2017) The impact of intrinsic anhydrite in an experimental calcium sulfoaluminate cement from a novel, carbon-minimized production process. Mater. Struct. 50, 144. https://doi.org/10.1617/s11527-017-1012-z. |
| 33. |
Brotherton, P.D.; Epstein, J.M.; Pryce, M.W.; White, A.H. (1974) Crystal Structure of Calcium Sulphosilicate Ca5(SiO4)2SO4. Aust. J. Chem. 27, 657–660. https://doi.org/10.1071/CH9740657. |
| 34. |
Shermanl, N.; Beretkal, J.; Santoro, L.; Valenti, G.L. (1995) Long-term behaviour of hydraulic binders based on calciumsulfoaluminate and calcium sulfosilicate. Cem. Concr. Res. 25 [1], 113–126. https://doi.org/10.1016/0008-8846(94)00119-J. |
| 35. |
Idrissi, M; Diouri, A.; Damidot, D.; Greneche, J.M.; Talbi, M.A.; Taibi, M. (2010) Characterisation of iron inclusion during the formation of calcium sulfoaluminate phase. Cem. Concr. Res. 40, 1314–1319. https://doi.org/10.1016/j.cemconres.2010.02.009. |
| 36. |
Idrissi, M; Diouri, A.; Talbi, M.A.; Sassi, O; Taibi, M.; Damidot, D. (2012) Hydration behavior of iron doped calcium sulfoaluminate phase at room temperature. MATEC Web of Conferences 2. https://doi.org/10.1051/matecconf/20120201005. |
| 37. |
Gallardo H., M.; Almanza R., J.M.; Cortés H., D.A.; Escobedo B., J.C. (2016) Mechanical and chemical behavior of calcium sulfoaluminate cements obtained from industrial waste. Revista ALCONPAT 6, 15–27, https://doi.org/10.21041/ra.v6i1.112. |
| 38. |
Taylor, H.F.W.; Famy, C.; Scrivener, K.L. (2016) Delayed ettringite formation. Cem. Concr. Res. 31, 683–693. https://doi.org/10.1016/S0008-8846(01)00466-5. |
| 39. |
Kaufmann, J.; Winnefeld, F.; Lothenbach, B. (2016) Stability of ettringite in CSA cement at elevated temperatures. Adv. Cem. Res. 28, 251–261. https://doi.org/10.1680/jadcr.15.00029. |
Figure 1. X-ray patterns of clinkers fired at 1120°C, 1220°C and 1320°C: a) Mixture A; b) Mixture B; c) Mixture C. Labels for clinker phases: B = β-belite; Y = ye’elimite; F = ferrite; G = gehlenite; T = ternesite; A = anhydrite; Q = quartz; L = lime.